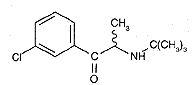
 Bupropion (amphebutamone), M 239,74 g/mol, is the active substance of Zyban®. It serves as retard tablet with sustained-release of bupropion for the nicotine curing. It can block the reuptake of the messenger materials norepinephrine and dopamine in the central nervous system. In which way this affects the nicotine craze, is at present not yet exactly known. Only 1 week after use of Zyban smoking should be stopped. After approximately 9 weeks the nicotine withdrawal is finished and Zyban is to be set off.
Bupropion (amphebutamone), M 239,74 g/mol, is the active substance of Zyban®. It serves as retard tablet with sustained-release of bupropion for the nicotine curing. It can block the reuptake of the messenger materials norepinephrine and dopamine in the central nervous system. In which way this affects the nicotine craze, is at present not yet exactly known. Only 1 week after use of Zyban smoking should be stopped. After approximately 9 weeks the nicotine withdrawal is finished and Zyban is to be set off.
As risk exists at one per mille of the patients the danger of convulsions. It is assumed that deaths by cardiac infarct and impact accumulation are related with the uptake of Zyban (source: Handbuch Medikamente - Sonderausgabe.
In the USA and Tschechien bupropion is sold under the brand Wellbutrin. From Spain the preparations Zyntabac and Quomem originate.
History
Bupropion was synthezised for the first time in 1966 and was certified in 1989 in the USA as antidepressive. Since, in the comparison with other antidepressives, the incidence of seizure was four times greater, it was first taken away from the market, however, it is now available as depot preparation (Wellbutrin SR and Wellbutrin XL), which releases bupropion at a slower rate.
In 1997, Zyban was approved for use as a smoking cessation aid. As adverse effects overweight or obesity are reported several times.

